Demonstrating the safety of implants with long leads (a pacemaker and a deep brain stimulator) during MRI scans in the Virtual Population.

Simulation of local RF heating of a pacemaker lead in a 1.5T MRI.
Multiple routing paths can be efficiently screened in parametric simulations with the help of precomputed libraries.
Implant MRI Safety
| Expertise and Infrastructure |
|
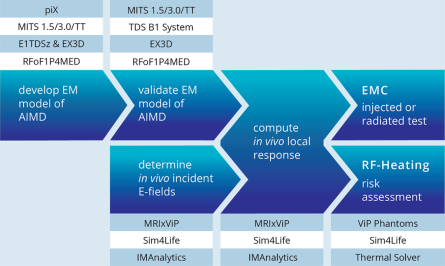
The IT’IS Foundation runs a dedicated group for customized research solutions in the area of MRI implant safety. IT’IS is your partner of choice as it (1) has pioneered the development of robust methods for the safety testing of Active Implantable Medical Devices (AIMD) and has contributed significantly to ISO/TS 10974, (2) applied these methods to a wide range of medical implants, (3) has detailed knowledge about the intricacies of regulatory agencies and the corresponding processes due to their active participation and membership in IEC/ISO standard groups, as well as (4) experience in interacting with the US Food and Drug Administration (FDA), and other regulators on numerous successful projects.
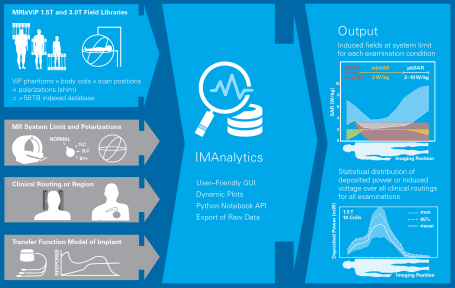
The group’s Yellow Submarine Laboratory is equipped with the most advanced tools including a PiX system, MITS-1.5T, -3T, -TT, -HFR, and -Gradient systems, the latest DASY6 RX90L with DASY52NEO licenses, and a wide range of specialized probes for E-field, SAR, H-field and voltage, including hardened probes designed specifically for MRI environments. The safety analysis as described in Tier 3 of ISO 10974 requires repeating the power deposition assessment for a wide range of computational models, for all the possible ways of clinical implantation in them, and for all possible exposure scenarios. With the MRIxViP exposure library and the IMAnalytics module of Sim4Life, this becomes a simple, reliable and traceable procedure. From the AIMD response model, the incident field distributions and the implants’ routing trajectories, IMAnalytics automatically performs the statistical analysis of power deposition at the tip of the AIMD lead in all possible scenarios. The results can be exported and included in regulatory submission reports.
IMAnalytics and MRIxViP are qualified by the FDA for MRI safety evaluations.
|
| Select Customized Research Projects of the Past Years |
|
| Contact |
|
We look forward to discussing with you how we can best support your R&D initiatives and regulatory submissions – simply call us at +41 44 245 96 96 or send us an email at customized@itis.swiss. |
RELEVANT PUBLICATIONS